INSEMA Findings and Discussion
Primary results of the INSEMA (Intergroup-Sentinel-Mamma) trial were presented at the 2024 San Antonio Breast Cancer Symposium and simultaneously published online in the NEJM on December 12, 2024.1, 2 The authors concluded that axillary sentinel lymph node surgery can be safely omitted in patients with clinically node-negative early breast cancer undergoing upfront breast-conserving surgery and whole breast radiation (breast-conserving therapy).
This study adds to the on-going discussion generated by the Sentinel Node vs Observation After Axillary Ultra-Sound (SOUND) trial3 on omission of axillary SLN surgery for patients with early-stage breast cancer. The highlights of this manuscript are summarized below:
- Prospective, randomized, non-inferiority trial conducted in 142 German and 9 Austrian sites between September 2015 – April 2019
- Enrolled patients ≥ 18 years of age with “early” invasive breast cancer, defined as:
- Tumor size ≤ 5 cm (clinical T1-T2)
- Clinically node-negative (cN0) based on clinical assessment and imaging
- All patients underwent routine axillary US and if suspicious (cortical thickness > 2.5 mm or no fatty hilum), a negative core or fine needle biopsy of the lymph node was required to be eligible.
- Underwent breast-conserving therapy (lumpectomy PLUS post-operative whole-breast radiotherapy)
- Patients were randomized 4:1 to SLN surgery vs no axillary surgery
- Primary end-point was invasive disease-free survival (iDFS)
- Clinical non-inferiority specified as the upper limit of the confidence interval for the hazard ratio below 1.271
- Per-protocol analysis cohort (4858 women – 3896 SLN surgery, 962 no axillary surgery)
- Patient and tumor features were well-balanced between groups: median age 62 (range 24-89), 90% cT1/78.6% pT1, 98.5% hormone receptor-positive, 3.6% HER2-positive, 3.6% grade 3, median FU 73.6 months (6.1 years)
- More patients in the SLN group received adjuvant chemotherapy (12.9% vs 10.4%)
- Non-inferiority criterion was met for iDFS: HR 0.91 (95% CI 0.73-1.14) for no axillary surgery compared to SLN surgery
- 5-year iDFS: 91.9% (95% CI 89.9%-93.5%) for no axillary surgery vs 91.7% (95% CI 90.8%-92.6%) for SLN surgery
- 5-year OS (Overall Survival): 98.2% (95% CI 97.1%-98.9%) for no axillary surgery vs 96.9% (95% CI 96.3%-97.5%) for SLN surgery
- Patients in the No axillary surgery group had a lower incidence of lymphedema, greater arm mobility, and less pain with arm/shoulder movement compared with the SLN surgery group.
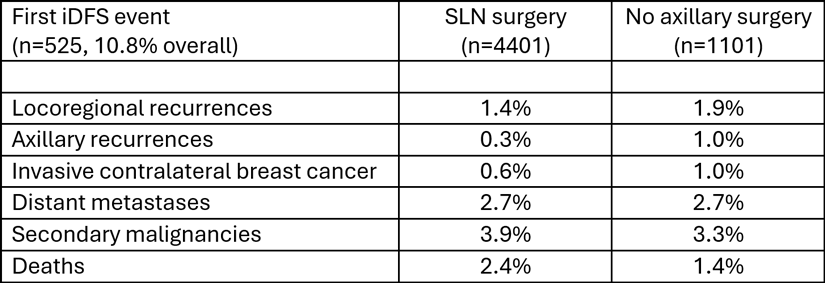
The authors concluded that omitting axillary sentinel lymph node surgery was not inferior to SLN surgery in clinically node-negative patients with early breast cancer having upfront breast-conserving therapy (lumpectomy plus whole-breast RT). SLN surgery omission was felt to be most suitable for patients > 50 years old with cancers that were hormone receptor-positive, HER2-negative, grade 1-2 and up to 2 cm in size (clinical T1 tumors).
This paper provides further data that axillary SLN surgery does not offer therapeutic benefit in the setting of breast conservation surgery with whole breast radiation. Similar to the SOUND trial, patients were eligible if they were deemed clinically node-negative based on clinical exam and pre-operative axillary ultrasound (+/- biopsy of any suspicious nodes).
These results should be applied with careful consideration. SLN surgery likely still has a role for guiding adjuvant therapies in some patients, including young patients (only 10.8% of the participants were less than 50 years of age), those with Grade 3 and/or HER 2 positive/triple-negative disease, where chemotherapy confers survival benefit for node-positive disease.
While the authors enrolled patients with tumors up to 5 cm in size, almost 80% of the tumors were less than 2 cm in size (pathologic T1). Extending omission of SLN surgery to T2 tumors thus requires guarded extrapolation. Identification of nodal positivity for HR-positive disease in pre menopausal and limited post menopausal patients may also influence the decision for adjuvant therapy (e.g. CDK4/6 inhibitor eligibility).
In the INSEMA trial, an eligibility requirement was post-operative whole-breast radiotherapy (partial breast irradiation was not allowed). As with the SOUND trial, some of the patients in INSEMA over the age of 65 with small tumors may be a candidate for partial breast irradiation or consideration of radiotherapy omission.
One limitation is that an event-driven analysis was initially planned, with an estimated 851 events needed for the trial to have 80% power to confirm noninferiority. Since only 525 events occurred, the trial ended up having less power than was planned.
Routine omission of axillary SLN surgery for patients with clinically node-negative early-stage invasive breast cancer having breast-conserving therapy will require careful, individualized implementation. There remain patients for whom the nodal information may either de-escalate radiation therapy or influence systemic therapy outside of available genomic testing. As always, multidisciplinary discussion is an important approach prior to practice-changing implementation of the SOUND and INSEMA trials.
- GS2-07: No axillary surgery versus axillary sentinel lymph node biopsy in patients with early invasive breast cancer and breast-conserving surgery: final primary results of the Intergroup-Sentinel-Mamma (INSEMA) trial. Reimer T, et al. San Antonio Breast Cancer Symposium 2024. Abstract #SESS-3619.
- Reimer T, Stachs A, Veselinovic K, et al. Axillary surgery in breast cancer – primary results of the INSEMA trial. N Eng J Med. 2024. doi:10.1056/NEJMoa2412063.
- Gentilini O, Botteri E, Sangalli C, et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes. The SOUND Randomized Clinical Trial. JAMA Oncol. 2023;9(11):1557-1564. doi:10.1001/jamaoncol.2023.3759.

